What Chemical Reactions Take Place In Catalytic Converters
All in all thats the whole process of how catalytic converters do actually work effectively. These honeycombs are covered with platinum group metals.
E Catalytic Converter A Catalytic Converter Design B Hydrocarbon Download Scientific Diagram
There are ceramic honeycombs in the catalytic converters.
What chemical reactions take place in catalytic converters. The catalyst is usually a mixture of at least two metals because one serves as a catalyst for the oxidation reaction and the other serves as a catalyst for the reduction reaction. Placed in the exhaust pipe and looks like a steel box. Convert carbon monoxide which is toxic into carbon dioxide convert nitrogen oxides which cause acid rain into nitrogen and oxygen A catalytic converter.
Catalytic converters are usually used with internal combustion engines fueled by petrol or diesel including lean-burn engines and sometimes on kerosene heaters and stoves. The reactions in catalytic converters. The catalytic converter is a sensitive device with precious metals coating the inside.
The two main types of catalytic. The historical meaning of its name comes from chemistry where a catalyst is an accelerator for a reaction. When adding the reduction agent ammonia formed by eg.
Many chemical reactions take place in the catalytic converter of a car. The catalytic converter usually lasts a long time. It is always designed to match the.
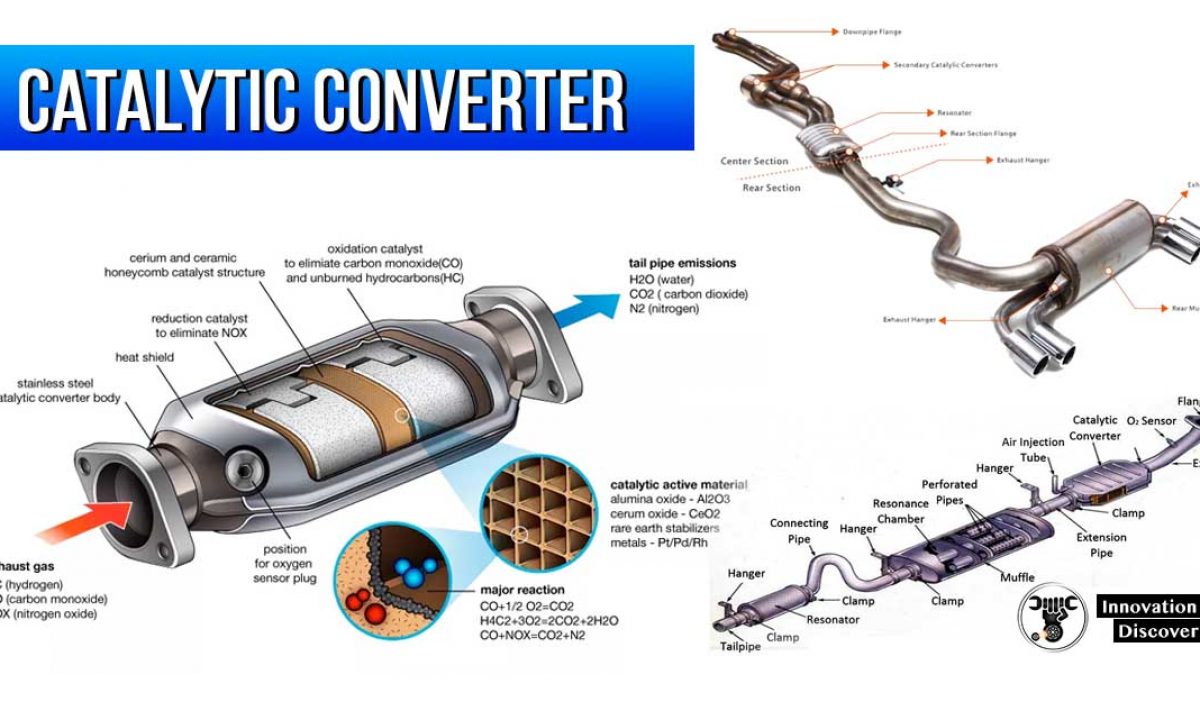
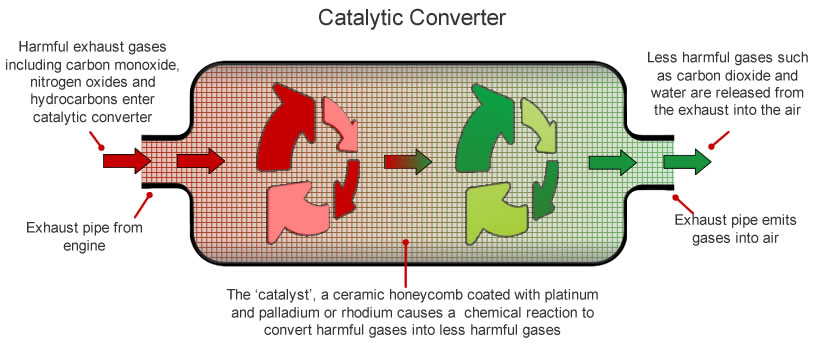
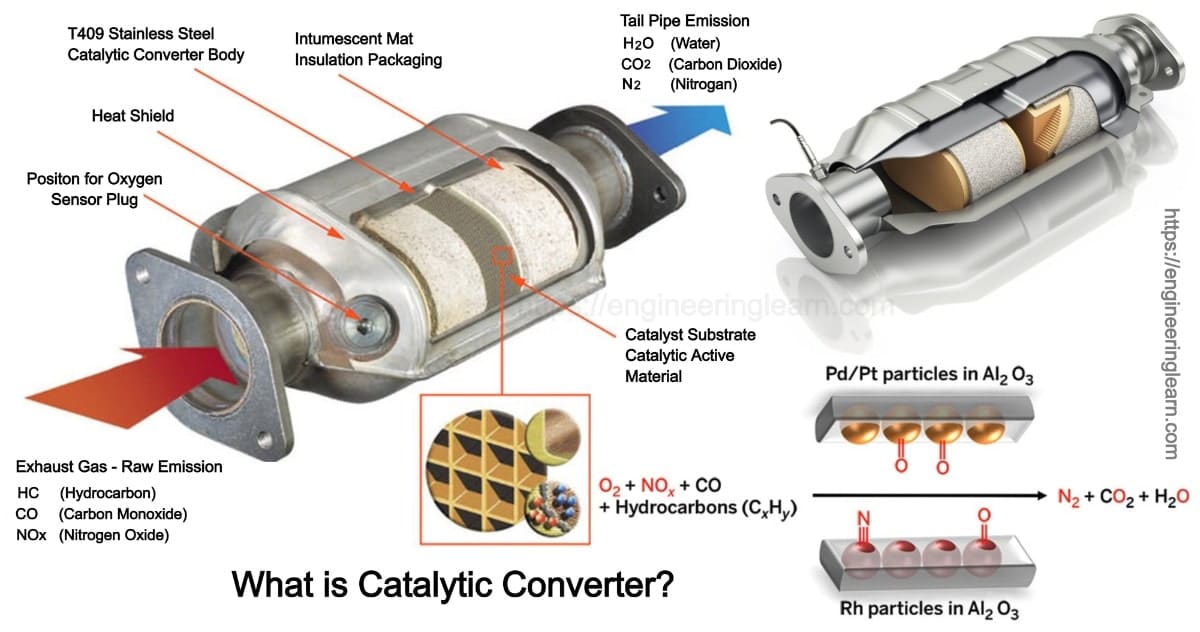
A catalytic converter is an exhaust emission control device that converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Modern catalytic converters are constructed from a tough heat-resistant ceramic material that is coated with catalysts like the noble metals mentioned above. Catalytic converters work primarily via catalytic chemical reactions.
However if lead is added to the fuel and is burned it leaves a residue that. Some less-effective converters carry out only the second two oxidation reactions so theyre called two-way catalytic converters After the catalyst has done its job what emerges from the exhaust is mostly nitrogen oxygen carbon dioxide and water in the form of steam. Since the converter is placed in-line between the exhaust manifold and the tail pipe the entirety of the vehicles exhaust are forced to pass through it.
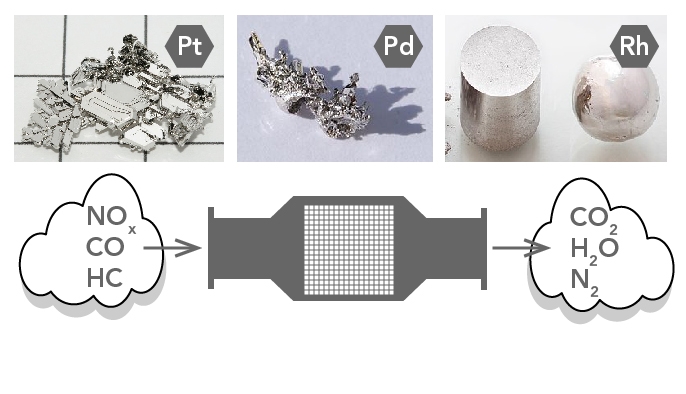
They use expensive metals like platinum palladium and rhodium as the heterogeneous catalyst. This is the reason they are installed next to the engine. Inside the device there are two substrates made of ceramic material.
CO and NO molecules diffuse over surface of catalytic converter They are adsorbed onto the surface and held in place where their bonds are weakend They react together- 2NO 2CO ----- N2 2CO2 N2 and CO2 are desorbed from the surface and diffuse out. Cause a desirable chemical reaction to take place in the exhaust flow. The Chemistry of the Catalytic Converter The oxidization of volatile hydrocarbons and CO2 is a simple process aided by a variety of chemical compounds.
In one of these reactions nitric oxide NO reacts with ammonia NH 3 to give nitrogen N 2 and water. Ceramic does not react with anything and is usually super-hot to allow reactions to take place. In summary it all comes down to these few simple processes.
The metals are deposited as thin layers onto a ceramic honeycomb. Most vehicles run on unleaded gasoline in which all the lead has been removed from the fuel. Without these metals the redox reactions cannot occur.
About 3-7 grams of additional rare metals in the cat Platinum Rhodium and Palladium react with the emissions. Nearly all converters use a combination of three chemicals -. Decomposition of urea nitrogen oxides are converted into harmless nitrogen and.
Essentially the catalytic converter is used to complete the oxidation process for hydrocarbon HC and carbon monoxide CO in addition to reducing oxides of nitrogen NOx back to simple nitrogen and carbon dioxide. The catalytic converter oxidizes all gases such as CO NO and HC to CO 2 NO 2 and CO 2 and water. Catalytic converters change poisonous molecules like carbon monoxide and various nitrogen oxides in car exhausts into more harmless molecules like carbon dioxide and nitrogen.
There are several substances and chemicals that inhibit the catalytic converter. With these metals chemical reaction takes place and the. Remember adsorbed with a d and desorbed.
It has a very simple system that performs filtering. When certain pollutant compounds pass through the monolith they interact with the catalyst and are converted into less harmful substances. The catalysts need to have a temperature above 300C for efficient performance.
Five thousand hours of life is common when ultralow sulfur fuel is used. Combustion produces hydrocarbons due to a lack of oxygen in the engine Reduction reactions make more oxygen form the oxygen and nitrogen that bond together during the burn. The function of the ceramic is to provide a large surface area for the.
In this case the catalytic converter receives exhaust gases a chemical reaction occurs. The magic of the catalytic converter takes place in the substrates. Typically catalytic converters require toxic air to pass through a honeycomb-shaped set of grills which increase the surface area of the catalyst material the gasses are exposed to.
The task of catalytic converters also known as catalysts is to remove toxic gases generated during the operation of the engines.
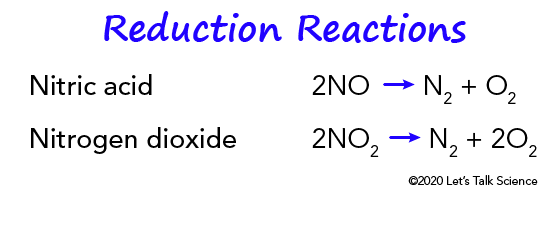
Catalytic Converters Let S Talk Science

Media Post Catalytic Converter Replacement Costs Best Selling Cars Blog
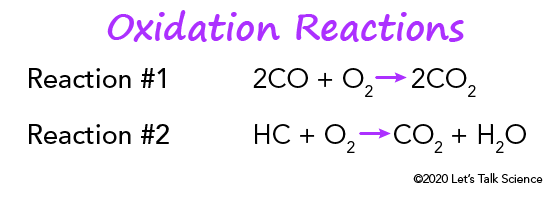
Catalytic Converters Let S Talk Science

What Is A Catalytic Converter Mahyaexhaust
Patent Picks Catalytic Converters

New Reduced Platinum Catalyst For Catalytic Converters Analyzing Metals
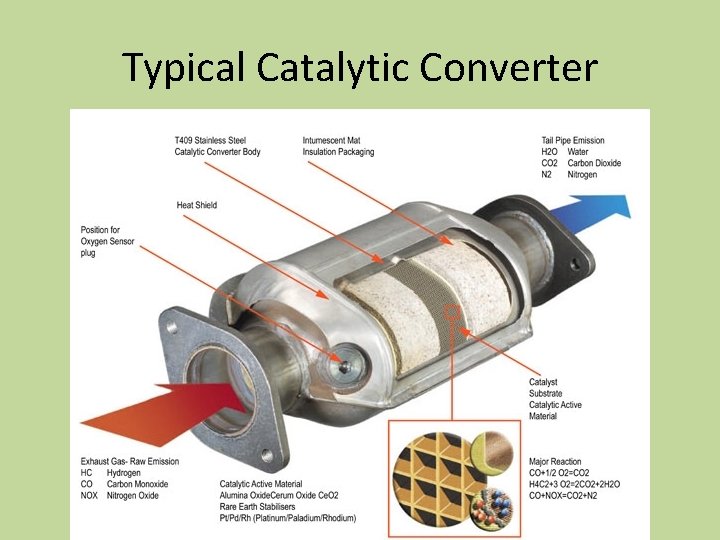
Chemical Kinetics Chapter 12 Chemical Kinetics Chemical Kinetics

What Does The Catalytic Converter Of An Automobile Really Do Quora
What Is Catalytic Converter In Cars How Does It Work Carbiketech

Geometry Of Catalytic Converter Of Motor Vehicle Download Scientific Diagram

Chemical Kinetics Chapter 12 Chemical Kinetics Chemical Kinetics

Schematic Of A Single Channel In A Dual Monolithic Catalytic Converter Download Scientific Diagram
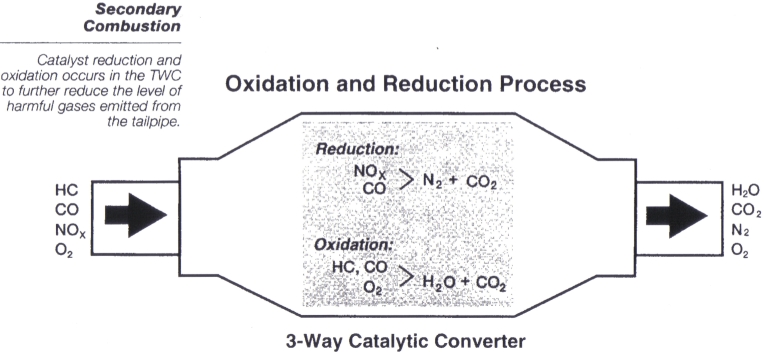
Understanding Catalitic Converters

What Are The Reactions That Occur In A Catalytic Converter In An Exhaust Quora

Structure Of Catalytic Converters Catalytic Converter Recycling
Post a Comment for "What Chemical Reactions Take Place In Catalytic Converters"